Acids in Coffee
#CaffeinatedTraining
#OilSlickCoffee
Presented: February 27th, 2018
Host: 5758 Coffee Lab
Press "h" for keyboard shortcuts for this presentation
"The acid content of green coffee bean is around 11%, mainly comprising citric, malic, chlorogenic and quinic acids, while roasted bean contains around 6% due to decreases in citric, malic and chlorogenic acids"1
"It is still unknown which acids are imperative to recreate the acidity experienced in coffee. It is generally understood that citric acid, malic acid, and acetic acid are the most important because they exist in high proportions..."2
Citric - Tastes like citrus fruit
Malic - Tastes like green apples
Acetic - Tastes like vinegar
Coffee pH ≈ 5
Neutral pH = 7.0
Acids in Coffee
- Organic acids are early products of plant metabolism
- Also are produced through degradation of more reduced chemical entities in plants
Put simply:
- Photosynthesis produces sugars, which are converted to energy. In the conversion process, organic acids are produced as by-products.
Put even more simply:
- In order for plants to form acids, they must have sugars.
Acids contribute a hydrogen ion, lowering pH.
Phosphoric acid is inorganic and requires a phosphate from the soil
Acids in Coffee
Other sources of acids in coffee
- Coffee plant/development → photosynthesis
- Processing → fermentation = acetic acids
- Roasting
- Brewing?
"Given the same roast level, coffees brewed at higher temperatures will exhibit higher extractable solubles, higher concentrations of total acids, and lower pH values."
"Since acids are water soluble, increases to water temperature produce higher levels of extraction"
The more time the coffee sits, the more acidic it will become
Higher acidity generally means higher cupping score
Acids in Coffee - Roasting
Roasting coffee beans creates a temperature gradient in the bean
Acetic acid is formed by the breakdown of sugars. Other acids are formed by the breakdown of other pre-existing compounds.
Heat accelerates chemical reactions and in this case the formation of acids.
Larger gradients therefore provide a greater difference in acid development with the beans.
Put simply: faster + hotter roast = greater difference between ground and whole color = more complex acidity
Draw bean and indicate heat on outer edges and temp + acid degradation gradients
Acids in Coffee
Citric and malic acids decompose at relatively high temps 176°C and 190°C respectively
Both are 'tart' but malic is smoother than citric.
Malic acid tends to be highly favored by coffee professionals when scoring a coffee.*
*Organic Acids Revisited, by Joseph Rivera, Roast Magazine, May/June 2015
Malic Acid
- Decomposes: ~190°C
- High concentration in apples
- Perceived sourness—sensation on the sides of the tongue
- Formed by plant before cherry harvest
- Regional humidity for plant increases acidity in cherry
- Generally, more fruitiness (as with wine) = more malic acid
- Typical medium roast will lose ~30% of original malic acid content
Acetic Acid
- Boiling point: 118 - 119°C
- High concentration in vinegar
- Mostly formed during roasting process
- Also a by-product of fermentation
- Can increase up to 25 times from initial green bean content
- Formed by breakdown of carbs (sugar) — therefore more prevalent in arabica VS robusta
- Volatile - readily detected by smell
- Maximum concentration at light roast.
Due to volatility, plays an important role in aroma.
Acetic acid levels peak at light-roast level and rapidly decreases with darker roasts.
Citric Acid
- Decomposes: ~176C
- Decreases at medium and dark roasts
- High concentration in limes, lemons, oranges, etc.
- One of the most predominant acids
- Contributes to perceived acidity
- Not formed or increased during roasting proces
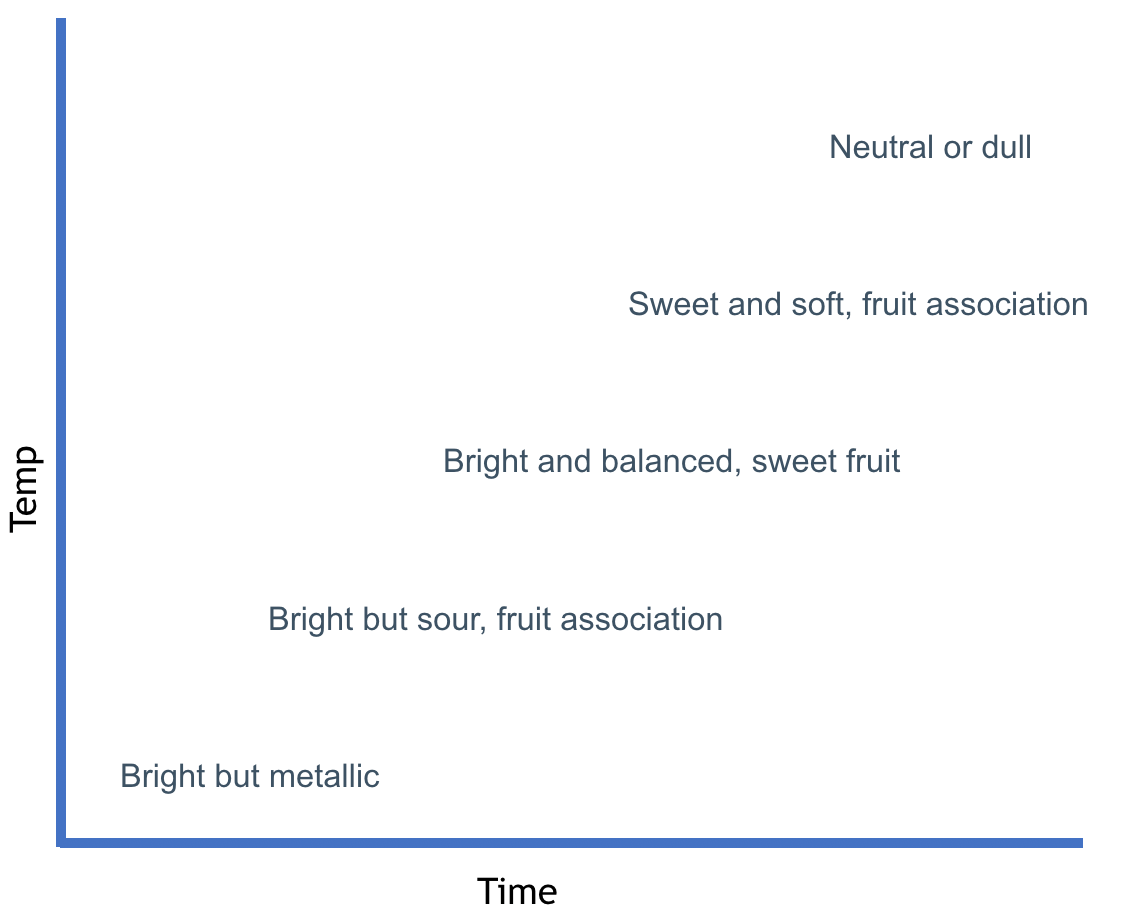
Acids in Coffee - Flavor Progression
Hoos, R. 2015. Modulating The Flavor Profile Of Coffee. Hoos Coffee
Value 4 Value
If you found this content useful, please consider supporting my work. I charge no set fee or price for providing this. You can help keep information like this openly accessible by matching the value you received in the content; value 4 value.
Ko-fi / Bitcoin Wallet: 32SW9kcAsJdZvQKBazhLUZBSD9YS8DDqe8
End of presentation