The Maillard Reaction
A key complex of chemical reactions
#CaffeinatedTraining
#OilSlickCoffee
Press "h" for keyboard shortcuts for this presentation
What is it? (40,000-foot view)
- Critical, chemical reaction in coffee roasting
- Occurs in many other foods as well
- Browning of foods such as bread, meat, etc
What is it? (better description, but still not great)
- Complex reaction of reactions
- Occurs at room temperature, but very slowly
- Occurs rapidly/readily at ≥ 140° - 165°C (280°-300°F)
- Produces hundreds of aromatics and flavonoids
- Produces caramel and melanoidins
- Higher temps = higher reaction velocity
- Higher reaction velocity = increased melanoidin production
- More sugars over amino acids also increase reaction rate
*Melanoidins Formed by Maillard Reaction in Food and Their Biological Activity, Food Eng Rev (2012) 4:203–223, ISSN 1866-7910
Primarily why second phase, starting at 300° is also referred to as the Maillard phase
Without the Maillard reaction, we aren’t roasting coffee
Maillard Reaction
The plot thickens
- Browning (caramel and melanoidin production) is the last step of the reaction1
- Therefore it is a useful indication of a phase-change in roasting process
- Removing chemically-free water is necessary in order to exceed 100°C/212°F (boiling point of water)2
- "The Maillard reaction will not cause browning in foods cooked with moist heat (boiling, steaming, poaching, stewing) because water cannot be heated above 212°F/100°C unless it is under pressure."3
- Water is a heatsink (large heat capacity)
- Fermentation of coffee may contribute amine-containing precursors for the Maillard reaction2
1 Melanoidins Formed by Maillard Reaction in Food and Their Biological Activity, Food Eng Rev (2012) 4:203–223, ISSN 1866-7910
2 http://luckypeach.com/opusculum-maillard-reactions/
3https://www.quora.com/Why-is-coffee-brown/answer/Peter-Baskerville#
If fermentation creates precursors for MR, this could expand our understanding of natural VS fully washed VS MR and flavor
Melanoidins
- Brown, fibrous polymers with a high molecular weight
- Largely responsible for a coffee’s body or mouthfeel
- “[I]ntimately associated in consumers’ minds with a high-grade product of desirable texture and flavour.”1
- Increasingly, studies indicate antioxidant effect1
- Also antimicrobial effects against pathogenic microorgs1
11 Melanoidins produced by the Maillard reaction: Structure and biological activity, Volume 128, Issue 3, Pages 573-822 (1 October 2011)
Not all that glitters is gold

Pyrite (Fools Gold) by pnjunction2007 from SENDAY/MIYAGI/JAPAN, Japan
[CC BY 2.0 (https://creativecommons.org/licenses/by/2.0)], via Wikimedia Commons
Not all that glitters is gold
Maillard reaction also produces the following
- Furfural (toxin)
- 5- hydroxymethylfurfural (possible carcinogen)
- Furans (possible carcinogen)
- Aldehyde (possible carcinogen)
Maillard reaction causes the loss of some nutrients (but we don’t drink coffee for its nutritional benefits)
The dose determines the poison
Melanoidins Formed by Maillard Reaction in Food and Their Biological Activity, Food Eng Rev (2012) 4:203–223, ISSN 1866-7910
California & Proposition 65
Coffee is safe to drink
Coffee drinking “shows reduction in cancers in certain humans, has not been shown to increase any cancers, and is particularly rich in cancer-chemo-preventive compounds.”
(But is not medicinal)
Joseph R. Landolph, Ph. D., https://oehha.ca.gov/media/downloads/crnr/coffeepeerreviewcomments091118.pdf
Leveraging the Maillard Reaction
Tracking the reaction
- It effectively starts at 140° - 165°C (280°-300°F)
- (coincides with the beginning of yellowing)
- The last step is browning via formation of caramel and melanoidins
- sugar caramelization occurs around 198°C/390°F
Therefore most of what happens after the MR is the creation of roasted flavors
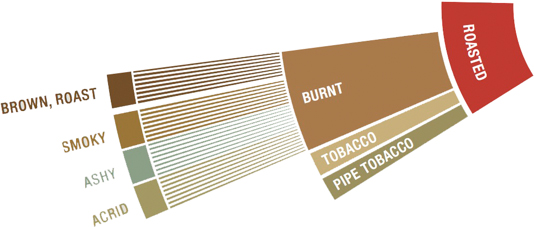
Knowing what flavors are caused and when empowers the roaster.
There is absolutely nothing wrong with darker roasts. Second crack is not a bad word.
There are coffees that do very well when roasted to a darker degree.
There are many customers who will prefer a darker roast.
To develop or avoid flavors, we need to know how and when they are formed.
Put simply
Longer Maillard reaction
= greater number of bi-products
= higher complexity + higher body
Not all that glitters is gold!
The price to pay for higher complexity and higher body
= lower clarity
Clarity is probably a bad term to use here. Complex, full-bodied coffees can be clear in what they are, i.e., they can have clarity.
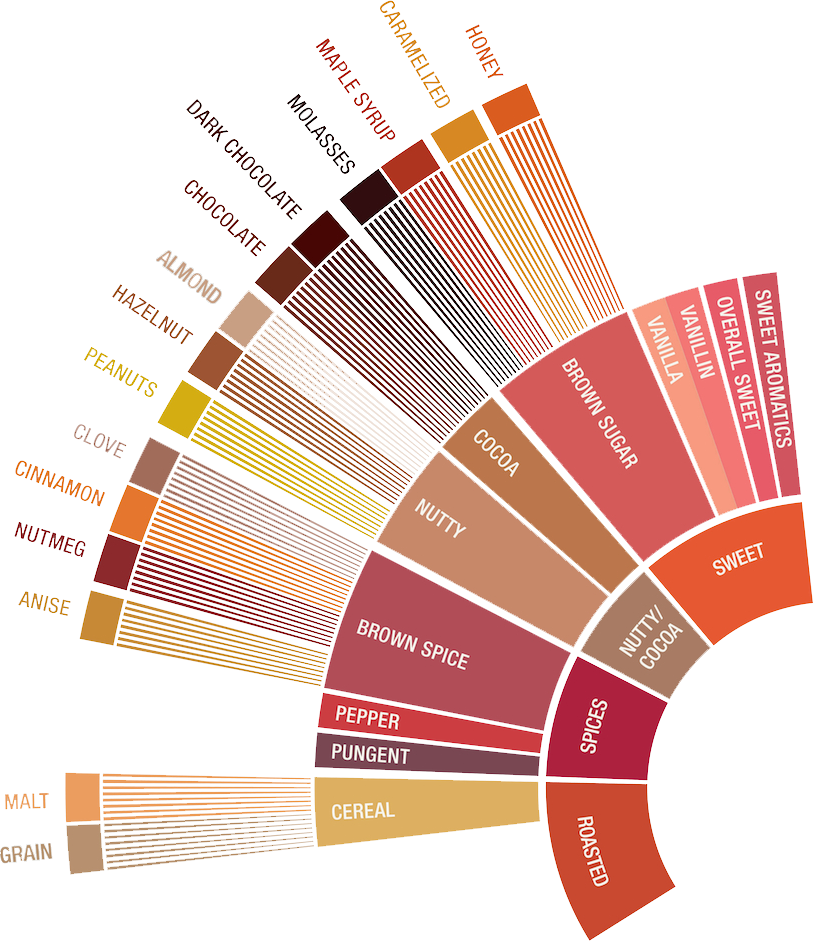
Maillard Reaction Cheat Sheet
- The Maillard phase consumes sugars and amino acids
- ...to produce melanoidins, which contribute to brownness of the roasted beans as well as the body of brewed or extracted coffee.
- A higher rate of heat application increases the reaction rate.
- The dominate flavors produced by the reaction are nutty, caramelly, chocolatey, malty flavors.
- If temps are equal, a longer reaction time = greater number of byproducts = higher complexity + higher body.
Value 4 Value
If you found this content useful, please consider supporting my work. I charge no set fee or price for providing this. You can help keep information like this openly accessible by matching the value you received in the content; value 4 value.
Ko-fi / Bitcoin Wallet: 32SW9kcAsJdZvQKBazhLUZBSD9YS8DDqe8
End of presentation